
The Koebner isomorphic phenomenon has been described in cutaneous reactions induced by drugs, such as antibiotics and chemotherapy. Due to the positive result of late skin test to clindamycin, oral challenge was not performed in our patient (3,5). Oral challenge tests are considered to be the gold standard to establish or exclude drug hypersensitivity. Positive late skin tests suggested delayed-type non-IgE-mediated allergic clindamycin hypersensitivity. were also positive (+ on day 2 and day 4). Late results of ID tests with clindamycin (1.5 and 15 mg/mL) were positive: erythematous infiltrated papules about 7×7 mm and 18×15 mm were observed at 24 hours and lasted until the 8th day. Prick and ID test results after 20 min were negative.
#Clindamycin skin rash Patch#
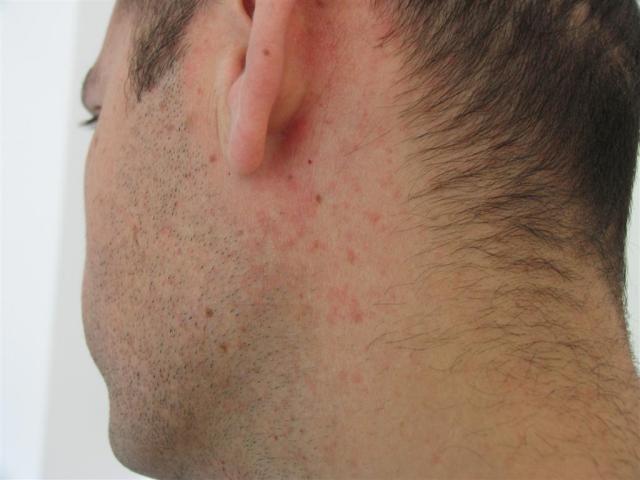
Patch tests were removed after the 2nd day, and late reactions were evaluated on day 2 and day 4. Prick and ID tests were assessed after 20 min and 24 hours, respectively. Other authors have reported that these concentrations do not seem to irritate the skin (3-6). Parenteral clindamycin preparations were used in therapeutic concentrations for prick tests (150 mg/mL) and dilutions in saline of 1/100 and 1/10 for the ID test. For patch testing, powder of the commercial capsules (Dalacin®) was diluted in petrolatum (pet.) and water (aq.), resulting in a final 1% clindamycin dilution. Eight weeks after clearance of the skin rash, informed consent was obtained in order to perform an allergological evaluation of clindamycin, including prick and intradermal (ID) tests on the forearm and patch tests on the upper back (2). On the 5th day of treatment, the rash had almost cleared with minimal desquamation (Figure 1, D). Clindamycin withdrawal was followed by prescription of a course of oral deflazacort, starting at 30 mg daily and tapering down during a 9-day period. No mucosal involvement was found, and laboratory studies showed no abnormalities. There was a striking vertical distribution of skin lesions along the SD on the lateral sides of the abdomen (Figure 1, C). Physical examination revealed an erythematous maculopapular eruption symmetrically distributed on the neck, abdomen, and back (Figure 1, A), with isolated lesions involving the proximal upper and lower limbs (Figure 1, B). General malaise (but not fever) was also reported. The skin rash first developed on her neck and back on the 3rd day of clindamycin oral treatment (300 mg every 6 hours), which was prescribed as antibiotic prophylaxis for a tooth implant.
Her past clinical history included hypertension, hypothyroidism, hyperuricemia, cholecystectomy, caesarean section, and endometriosis-related abdominal surgery, and she was taking levothyroxine, allopurinol, imidapril, and omeprazole. A 47-year-old woman was referred to our clinic for pruritic cutaneous lesions which had started 6 days earlier. We report the case of a patient with a maculopapular rash triggered by clindamycin who developed cutaneous lesions on striae distensae (SD). Cutaneous adverse reactions are usually maculopapular exanthemas, although hypersensitivity syndrome, acute generalized exanthematous pustulosis, and Stevens-Johnson syndrome have also been reported (1). Clindamycin is a lincomycin-derived antibiotic useful for the treatment of anaerobic and Gram-positive aerobic bacterial infections.


 0 kommentar(er)
0 kommentar(er)
